
Next, a search of electrons is required by a single CH4 molecule to reach a stable condition.
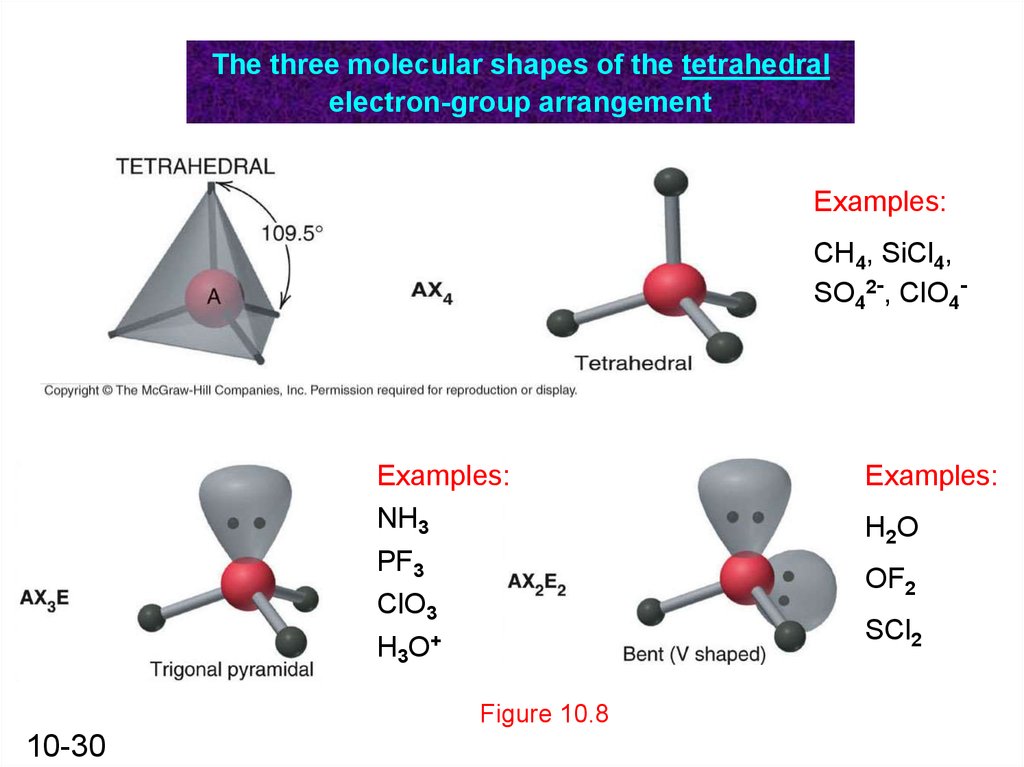
Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step.įirstly, look for the total number of valence electrons required by a single CH4 molecule, which is sixteen. The lewis structure of carbon and hydrogen atom says- to form a single CH4 molecule, a total of eight valence electrons participate in the shared bonding to fulfill the need of eight more valence electrons. The lewis structure of CH4 is drawn to fulfill the need of valence electrons by all the atoms. If we follow this rule, it is much easier to see that carbon has a dearth of four valence electrons whereas, hydrogen needs only one valence electron. This rule says the maximum valence electrons that can be drawn around an atom are eight. Whereas, on the other hand, the atomic number of the hydrogen atom is one that makes its electronic configuration 1s1.Īs there is a dearth of only one electron, the number of valence electrons in a hydrogen atom is one. Due to this, the number of valence electrons in the carbon atom has been four. So, the electronic configuration of the carbon will be 1s2 2s2 2p2.Īs the p shell needs to accommodate a total of six electrons, there is a dearth of four electrons. To know the number of valence electrons in a carbon atom, first, it is crucial to find its atomic number which is six. There can be a maximum of eight valence electrons in an atom. These are the electrons that participate in the bond formation by either getting donated or accepted between the atoms. We offer tutoring programs for students in K-12, AP classes, and college.Valence electrons are those electrons that take participation in the bond formation and exist in the outermost shell of an atom. SchoolTutoring Academy is the premier educational services company for K-12 and college students. Interested in chemistry tutoring services? Learn more about how we are assisting thousands of students each academic year. Polar molecules are attracted by electrical charge, and line up in the charge.įigure 3: Sulfur hexafluoride (SF 6) is an example of an octahedral molecular shape. Not all molecules have polarity, but those that do are negatively charged at one pole and positively charged at the other pole, because of the molecular bonds that have formed. Other shapes include the T-shaped molecule chlorine trifluoride (ClF 3), octahedral-shaped molecules such as sulfur hexafluoride (SF 6), and other symmetrical and asymmetrical variations. The hydrogen atoms in a water molecule (H 2O) are joined to the oxygen atom in a bent shape, rather than a straight line. The carbon atom is the pink one in the center, surrounded by 4 white hydrogen atoms. The atoms in ammonia (NH 3) are arranged in a three-dimensional pyramid.įigure 2: Three-dimensional tetrahedral molecular shape, such as methane (CH 4). However, its atoms are not arranged in a two-dimensional square, but in a three-dimensional tetrahedral shape, similar to a tripod, called a tetrahedral shape. A molecule such as methane (CH 4) has 4 atoms of hydrogen joined to one atom of carbon in the center. Some molecular shapes are a simple arrangement, but the atoms are arranged in three dimensions rather than the two dimensional flat molecular shapes. In a molecule of boron trichloride (BCl 3), each chlorine atom is bonded to the boron atom in the center.įigure 1: Boron trichloride (BCl 3) is an example of a trigonal planar molecule. A trigonal planar molecule has three bonds in a Y-shape. All molecules that contain only two atoms have a linear bond. The chemical formula for carbon dioxide doesn’t reflect its structure, but the type of strong atomic bonds does. The oxygen atoms are double-bonded with the carbon atom. Some molecules are arranged with atoms in a straight line, such as carbon dioxide (CO 2). Some atoms share electrons, and other electron pairs are not bonded. This results in the distinctive shapes of small molecules, in order to preserve molecular bonding. To compensate for the repulsion of like charges, the pairs of electrons in a molecular bond are as far apart from each other as they can be.

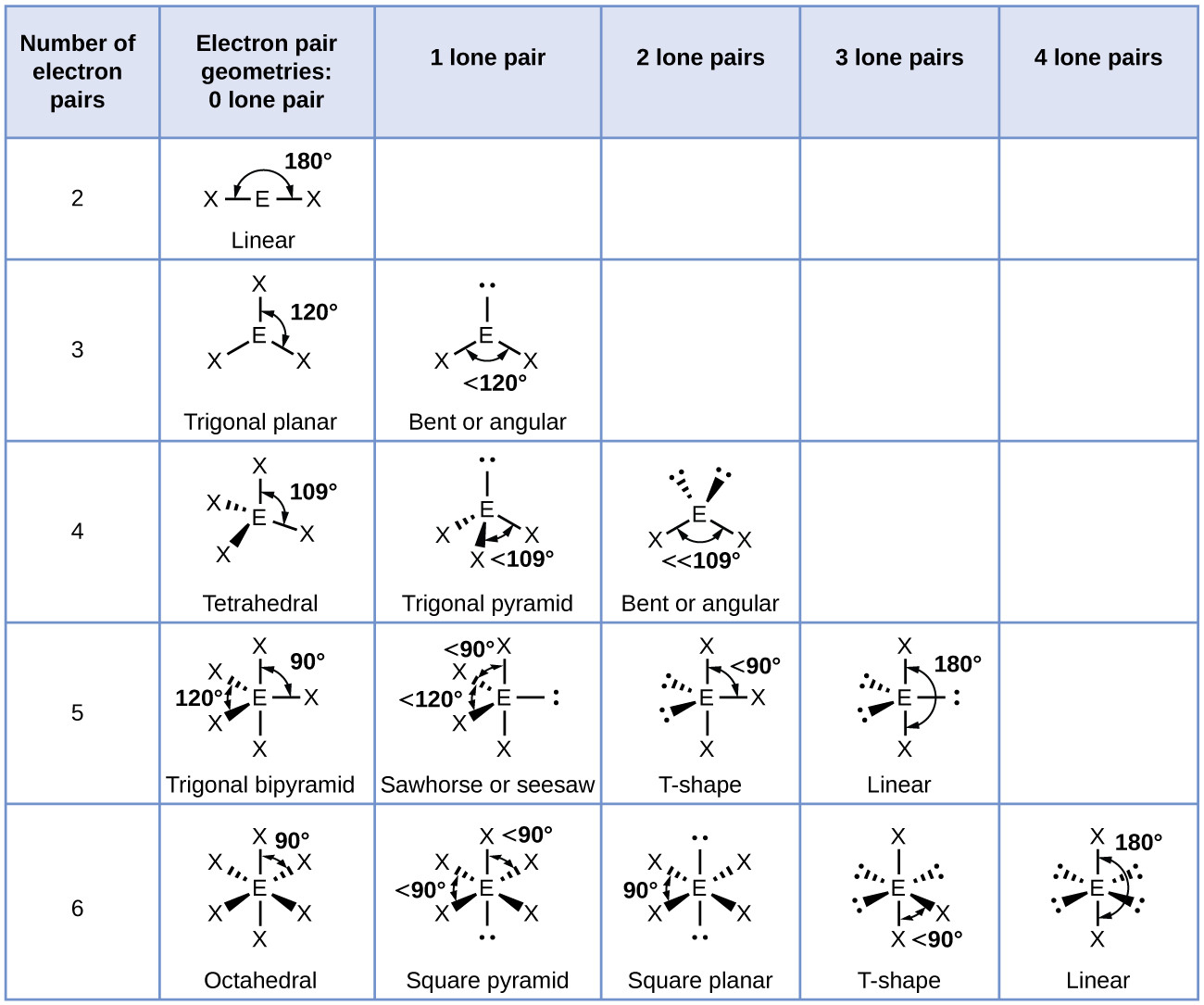
Electron pairs have the same negative charge, but the electrons share valances in molecular bonding. The shape of many small molecules can be predicted by the valence-shell electron pair repulsion theory or VSEPR for short. Some molecules also exhibit polarity, which gives them a distinct positive or negative charge. Symmetrical molecules tend to follow basic structural shapes, such as linear, planar, tetrahedral, pyramidal, and bent.


 0 kommentar(er)
0 kommentar(er)
